Textbook Question
Which of the following structures have no electrical charge?
a. Protons
b. Electrons
c. Neutrons
d. Ions
602
views

 Verified step by step guidance
Verified step by step guidance
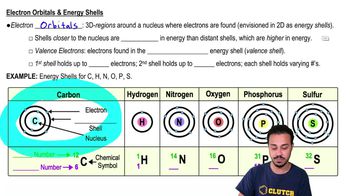


Which of the following structures have no electrical charge?
a. Protons
b. Electrons
c. Neutrons
d. Ions
List three main types of chemical bonds, and give an example of each.
In the following molecule, label a portion that shows only primary structure; label two types of secondary structure; circle the tertiary structure.
The atomic mass of an atom most closely approximates the sum of the masses of all its __________.
a. protons
b. isotopes
c. electrons
d protons and neutrons