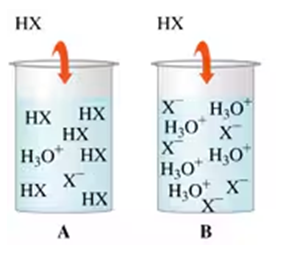
Textbook Question
How many grams of Al(OH)3 are required to neutralize 150. mL of stomach acid with a pH of 1.5?
825
views

 Verified step by step guidance
Verified step by step guidance


How many grams of Al(OH)3 are required to neutralize 150. mL of stomach acid with a pH of 1.5?
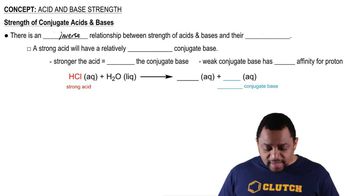
Identify each of the following as an acid or a base:
d. HI
Identify each of the following as an acid or a base:
b. H2SO3
Sometimes, during stress or trauma, a person can start to hyperventilate. Then the person might breathe into a paper bag to avoid fainting.
a. What changes occur in the blood pH during hyperventilation?
<IMAGE>
Sometimes, during stress or trauma, a person can start to hyperventilate. Then the person might breathe into a paper bag to avoid fainting.
b. How does breathing into a paper bag help return blood pH to normal?
<IMAGE>
Identify each of the following as an acid, base, or salt, and give its name:
e. H2CO3