Textbook Question
Avobenzone is a common ingredient in sunscreen. Its structural formula is shown.
b. What is the molecular formula and molar mass of avobenzone?
1056
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones
Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones Problem 41
Problem 41 Verified step by step guidance
Verified step by step guidance

Avobenzone is a common ingredient in sunscreen. Its structural formula is shown.
b. What is the molecular formula and molar mass of avobenzone?
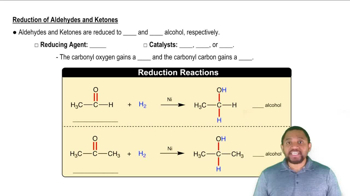
A compound called resveratrol is an antioxidant, found in the skin of grapes. Identify the functional groups in resveratrol.
<IMAGE>
A compound called cinnamaldehyde is found in cinnamon. Identify the functional groups in cinnamaldehyde.
<IMAGE>
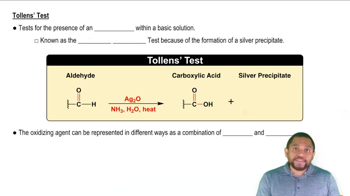
Which of the following will give a positive Tollens' test?
a. 1-propanol
b. 2-propanol
c. hexanal
Give the IUPAC name for each of the following alcohols and phenols:
a.
Give the IUPAC name for each of the following alcohols and phenols:
c.