Textbook Question
Write equations that show the processes that describe thefirst, second, and third ionization energies of a chlorineatom. Which process would require the least amount ofenergy?
1251
views
1
rank

 Verified step by step guidance
Verified step by step guidance

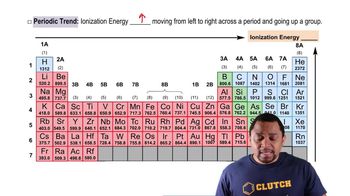

Which element has the highest second ionization energy: Li, K, or Be?
(a) What is the general relationship between the size of an atom and its first ionization energy?
(b) Which element in the periodic table has the largest ionization energy? Which has the smallest?