Identify the specific element that corresponds to each of the following electron configurations and indicate the number of unpaired electrons for each: (a) 1s22s2 (b) 1s22s22p4

(a) What does the term paramagnetism mean? (b) How can one determine experimentally whether a substance is paramagnetic? (c) Which of the following ions would you expect to be paramagnetic: O2+ , N22 -, Li2+ , O22 - ? For those ions that are paramagnetic, determine the number of unpaired electrons.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Paramagnetism
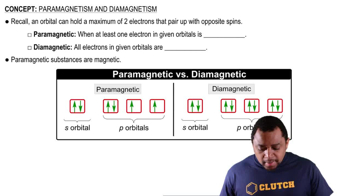
Experimental Determination of Paramagnetism

Unpaired Electrons and Ions

Identify the specific element that corresponds to each of the following electron configurations and indicate the number of unpaired electrons for each: (c) 3Ar44s13d5
Identify the specific element that corresponds to each of the following electron configurations and indicate the number of unpaired electrons for each: (d) 3Kr45s24d105p4.
The following do not represent valid ground-state electron configurations for an atom either because they violate the Pauli exclusion principle or because orbitals are not filled in order of increasing energy. Indicate which of these two principles is violated in each example. (a) [Ne]3s23p63d5 (b) [Xe]6s3 (c) 1s23s1.
The following electron configurations represent excited states. Identify the element and write its ground-state condensed electron configuration. (b) 3Ne43s13p44p1.
Consider the two waves shown here, which we will consider to represent two electromagnetic radiations: (a) What is the wavelength of wave A?
