Textbook Question
What is the difference between atomic oxygen and molecular oxygen?
786
views
 Verified step by step guidance
Verified step by step guidance
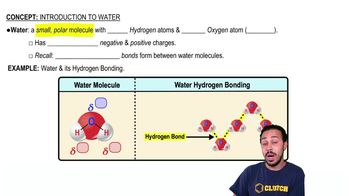
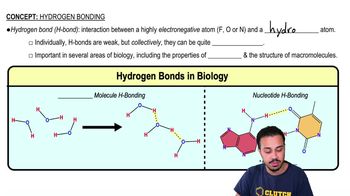
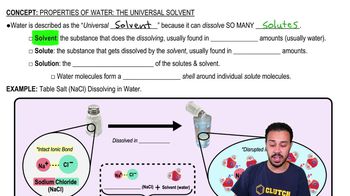
What is the difference between atomic oxygen and molecular oxygen?
List three main types of chemical bonds, and give an example of each.
Name five properties of water that are vital to life.
A nucleic acid containing the base uracil would also contain __________ sugar.
In the following molecule, label a portion that shows only primary structure; label two types of secondary structure; circle the tertiary structure.
Shown is the amino acid tryptophan. Put the letter “C” at the site of every carbon atom. Label the amino group, the carboxyl group, and the side group.