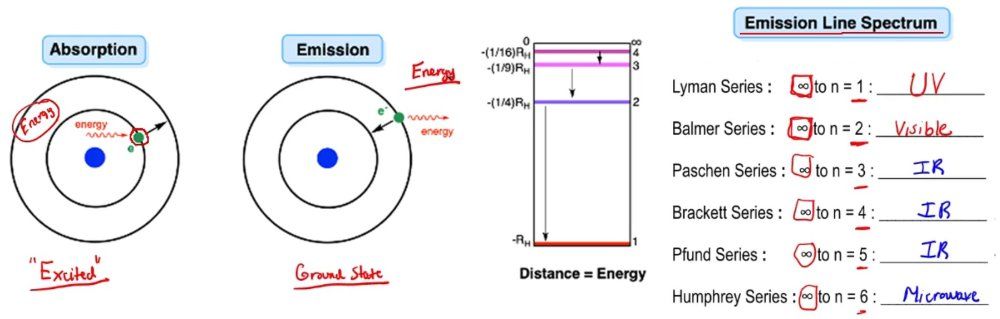
The concepts of emission and absorption are fundamental in understanding atomic behavior and energy transitions. Absorption occurs when an electron or atom takes in excess energy, allowing it to move to a higher energy state, often referred to as an excited state. For instance, in Bohr's model of the hydrogen atom, an electron in the first shell can absorb energy from its surroundings, promoting it to a higher shell. However, this excess energy cannot be retained indefinitely; eventually, the electron will release this energy and return to its ground state.
When discussing emissions, we can refer to emission line spectra, which are produced when an electron transitions from a higher energy shell back to a lower one. The periodic table currently recognizes seven electron shells, but this number may increase as new elements are discovered or synthesized. Each transition corresponds to a specific series of emissions. For example, when an electron drops from a higher shell to the first shell, it produces the Lyman series, which falls within the ultraviolet (UV) spectrum. The Balmer series occurs when an electron transitions to the second shell, emitting visible light. Other series, such as the Paschen and Brackett series, correspond to transitions to the third and fourth shells, respectively, and fall within the infrared (IR) spectrum. The Humphreys series is associated with transitions closer to the microwave region.
The energy associated with these transitions can be quantified using the Rydberg formula for potential energy, expressed as:
\( E = -1.8 \times 10^{-18} \, \text{J} \times \frac{1}{n^2} \)
where \( n \) represents the principal quantum number of the shell. As the shell number increases, the distance between shells decreases, which affects the energy released during transitions. A larger drop from a higher shell to a lower shell results in greater energy release.
Emission and absorption spectra are crucial for identifying elements. An emission spectrum displays bright lines against a dark background, representing the frequencies of electromagnetic radiation emitted as electrons fall to lower energy states. Conversely, an absorption spectrum features dark lines on a colored background, indicating the specific wavelengths absorbed by electrons as they transition to higher energy states. The key difference lies in the source of energy: emission spectra originate from excited atoms, while absorption spectra result from light passing through a medium containing atoms that absorb specific wavelengths.
In summary, absorption involves the intake of energy to elevate an electron to a higher state, while emission is the release of that energy as the electron returns to its ground state. Each transition corresponds to a specific series within the electromagnetic spectrum, highlighting the intricate relationship between energy levels and atomic structure.