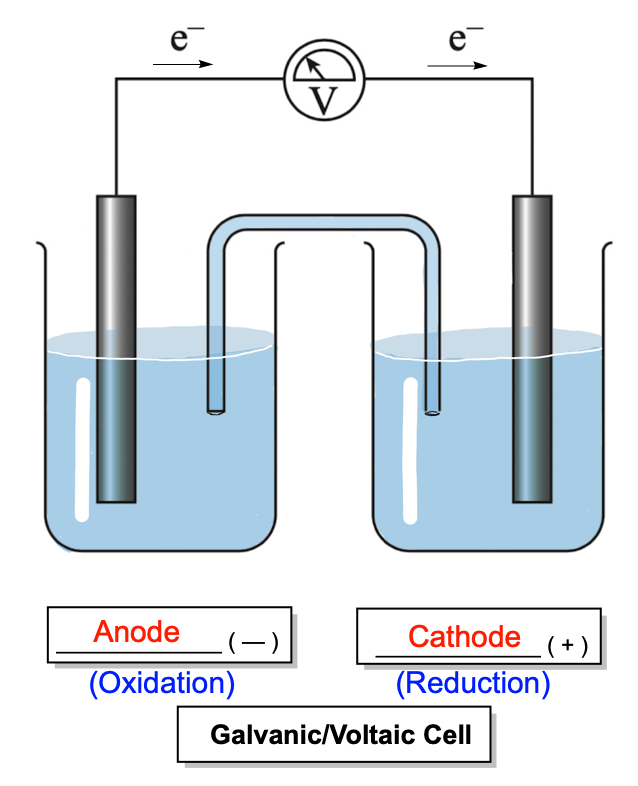
Galvanic or voltaic cells are spontaneous electrochemical cells that generate electricity through redox reactions. In these cells, the negative electrode is known as the anode, while the positive electrode is referred to as the cathode. When the cell discharges all its electricity, it is considered a dead battery.
In a typical galvanic cell, two half-reactions occur: at the cathode, copper ions (\( \text{Cu}^{2+} \)) gain electrons to form solid copper (\( \text{Cu} \)), represented by the equation:
\[ \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \]
This indicates that three moles of copper ions absorb six moles of electrons to produce three moles of solid copper. Conversely, at the anode, solid chromium (\( \text{Cr} \)) loses electrons to form chromium ions (\( \text{Cr}^{3+} \)), as shown in the equation:
\[ \text{Cr} \rightarrow \text{Cr}^{3+} + 3e^- \]
Here, two moles of chromium lose six electrons to produce two moles of chromium ions. The two compartments of the cell are connected by a salt bridge, which contains neutral ions, typically chloride or nitrate ions. These ions facilitate the flow of charge, with negative ions moving toward the anode and positive ions moving toward the cathode.
In galvanic cells, oxidation occurs at the anode, where electrons are released, while reduction occurs at the cathode, where electrons are accepted. The movement of electrons from the anode to the cathode generates an electric current, which can be measured using a voltmeter. The efficiency of this process depends on the ionization energy of the anode and the electron affinity of the cathode. A low ionization energy at the anode allows for easier electron loss, while a high electron affinity at the cathode ensures effective electron attraction.
Over time, the anode loses mass as it dissolves into the solution, while the cathode gains mass as copper ions deposit onto its surface, a process known as plating. To maintain efficient operation, the concentration of positive ions at the anode should be low, while the concentration of positive ions at the cathode should be high. This balance ensures that electrons continue to flow toward the cathode, as like charges repel each other.
Each half-reaction in a galvanic cell is associated with a standard cell potential, which indicates the likelihood of reduction occurring. A higher standard cell potential suggests a stronger oxidizing agent, while a lower potential indicates a stronger reducing agent. Understanding these principles allows for the exploration of various galvanic cell configurations and their respective half-reactions.