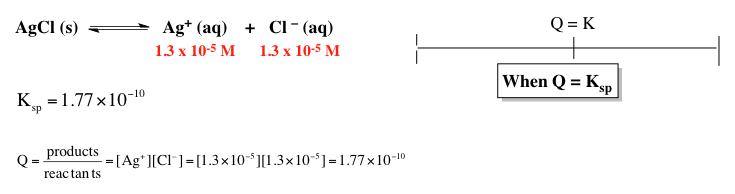
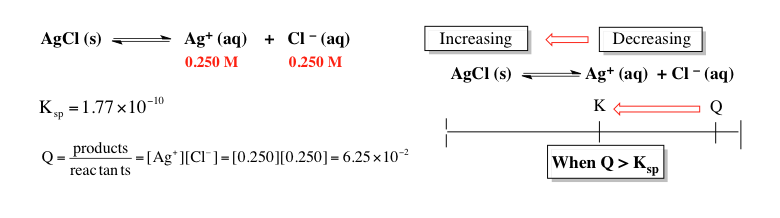
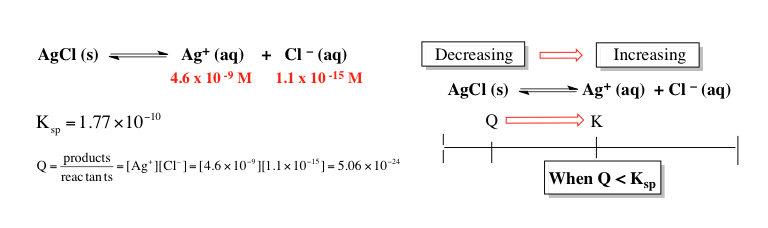
To determine which ionic compound has the highest molar solubility in pure water, the first step is to analyze how many ions each compound dissociates into when dissolved. For example, cobalt(II) hydroxide dissociates into one cobalt(II) ion and two hydroxide ions, totaling three ions. Strontium phosphate breaks down into three strontium ions and two phosphate ions, resulting in five ions. Lead(II) chloride yields one lead(II) ion and two chloride ions, also totaling three ions. Silver cyanide dissociates into one silver ion and one cyanide ion, giving two ions. Finally, lead(II) sulfate breaks down into one lead(II) ion and one sulfate ion, resulting in two ions.
Next, we compare the compounds with the same number of ions. Cobalt(II) hydroxide and lead(II) chloride both yield three ions. By examining their solubility product constants (Ksp), we find that lead(II) chloride has a higher Ksp value of 1.60 × 10-5, indicating it is more soluble than cobalt(II) hydroxide. Therefore, lead(II) chloride has a greater molar solubility.
For silver cyanide and lead(II) sulfate, both dissociate into two ions. The Ksp for silver cyanide is 1.0 × 10-8, while lead(II) sulfate has a Ksp of 1.82 × 10-8. This shows that lead(II) sulfate is more soluble, thus having a higher molar solubility than silver cyanide.
Now, we focus on strontium phosphate, lead(II) chloride, and lead(II) sulfate, which dissociate into different numbers of ions. To find the molar solubility (denoted as x) for strontium phosphate, we set up the expression based on its dissociation: Ksp = [Sr2+]3 × [PO43-]2. This results in Ksp = (3x)3 × (2x)2 = 108x5. Given Ksp = 4.0 × 10-28, we solve for x, yielding a molar solubility of approximately 1.30 × 10-6 M.
For lead(II) chloride, the dissociation gives Ksp = [Pb2+] × [Cl-]2 = x × (2x)2 = 4x3. With Ksp = 1.60 × 10-5, solving for x results in a molar solubility of about 0.0159 M.
Lastly, for lead(II) sulfate, we have Ksp = [Pb2+] × [SO42-] = x2. Given Ksp = 1.82 × 10-8, solving for x gives a molar solubility of approximately 1.35 × 10-4 M.
Comparing the molar solubilities, lead(II) chloride has the highest value, followed by lead(II) sulfate, and then strontium phosphate. Thus, the compound with the highest molar solubility in pure water is lead(II) chloride, as it has the largest x value.
In summary, the strategy for determining molar solubility involves first identifying the number of ions produced by each ionic compound, comparing their Ksp values for those that dissociate into the same number of ions, and calculating the molar solubility for those that dissociate into different numbers of ions. This systematic approach allows for the identification of the most soluble compound.