21. Kinetic Theory of Ideal Gases
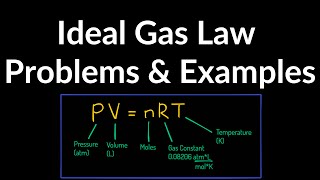
The Ideal Gas Law
Problem 18a
Textbook Question
Textbook QuestionA large cylindrical tank contains 0.750 m^3 of nitrogen gas at 27°C and 7.50 * 10^3 Pa (absolute pressure). The tank has a tight-fitting piston that allows the volume to be changed. What will be the pressure if the volume is decreased to 0.410 m^3 and the temperature is increased to 157°C?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
164
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos