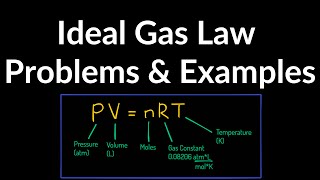
21. Kinetic Theory of Ideal Gases
The Ideal Gas Law
Learn with other creators
Practice this topic
- Multiple Choice
3 moles of an ideal gas fill a cubical box with a side length of 30cm. If the temperature of the gas is 20°C, what is the pressure inside the container?
1050views6rank - Multiple Choice
Hydrogen gas behaves very much like an ideal gas. If you have a sample of Hydrogen gas with a volume of 1000 cm3 at 30°C with a pressure of 1 × 105 Pa, calculate how many hydrogen atoms (particles) there are in the sample.
2011views8rank1comments - Multiple Choice
A balloon contains 3900cm3 of a gas at a pressure of 101 kPa and a temperature of –9°C. If the balloon is warmed such that the temperature rises to 28°C, what volume will the gas occupy? Assume the pressure remains constant.
953views4rank - Multiple ChoiceA sample of gas is heated in a closed container with a volume of from an initial temperature and pressure of and to a final temperature of . What is the final pressure?788views
- Textbook Question
A constant-volume gas thermometer registers an absolute pressure corresponding to mm of mercury when in contact with water at the triple point. What pressure does it read when in contact with water at the normal boiling point?
1273views - Textbook Question
The pressure of a gas at the triple point of water is atm. If its volume remains unchanged, what will its pressure be at the temperature at which CO2 solidifies?
1637views - Textbook Question
A large cylindrical tank contains m3 of nitrogen gas at °C and Pa (absolute pressure). The tank has a tight-fitting piston that allows the volume to be changed. What will be the pressure if the volume is decreased to m3 and the temperature is increased to °C?
755views - Textbook Question
You have several identical balloons. You experimentally determine that a balloon will break if its volume exceeds L. The pressure of the gas inside the balloon equals air pressure ( atm). If the air inside the balloon is at a constant °C and behaves as an ideal gas, what mass of air can you blow into one of the balloons before it bursts?
888views