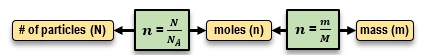
In physics and thermodynamics, understanding the relationship between mass, moles, and the number of particles is crucial for calculations involving gases and other materials. The mole, represented by the symbol n, is a unit that quantifies the amount of substance. One mole corresponds to 6.022 × 1023 particles, a value known as Avogadro's number, denoted as NA. This concept is similar to a dozen, which signifies 12 items; thus, a mole signifies 6.022 × 1023 of any given particle, whether atoms, molecules, or even dollars.
To convert between mass, moles, and the number of particles, specific equations are utilized. The number of moles can be calculated from the number of particles using the formula:
n = \frac{N}{NA}
where N is the number of particles. For example, if you have 8.33 × 1037 carbon atoms, you can find the number of moles by dividing this number by Avogadro's number, resulting in approximately 1.38 × 1014 moles of carbon.
Conversely, to find the mass from the number of moles, the equation used is:
m = n × M
where M is the molar mass of the substance. For instance, if you have 2.35 moles of aluminum with a molar mass of 26.98 g/mol, the mass can be calculated as:
m = 2.35 × 26.98 = 63.4 g
When starting with mass and needing to find the number of particles, the process involves two steps. First, convert mass to moles using:
n = \frac{m}{M}
For example, if you have 24 g of water (H2O) with a molar mass of 18.02 g/mol, the number of moles is:
n = \frac{24}{18.02} ≈ 1.33
Next, convert moles to particles using:N = n × NA
Thus, N = 1.33 × 6.022 × 1023 ≈ 8.01 × 1023 particles of H2O.
By mastering these conversions and understanding the relationships between mass, moles, and particles, you can effectively navigate thermodynamic calculations and deepen your comprehension of material properties.


 1 student found this helpful
1 student found this helpful