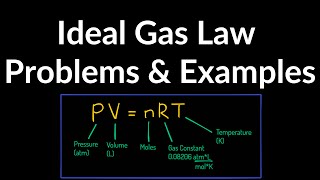
21. Kinetic Theory of Ideal Gases
The Ideal Gas Law
Multiple Choice
Multiple ChoiceA balloon contains 3900cm3 of a gas at a pressure of 101 kPa and a temperature of –9°C. If the balloon is warmed such that the temperature rises to 28°C, what volume will the gas occupy? Assume the pressure remains constant.
A
0.012 m3
B
0.0041 m3
C
0.39 m3
D
0.0044 m3
328
views
1
rank
Related Videos
Related Practice
Showing 1 of 10 videos