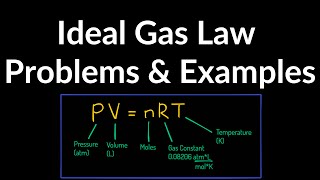
21. Kinetic Theory of Ideal Gases
The Ideal Gas Law
Problem 18e
Textbook Question
Textbook QuestionA 20.0-L tank contains 4.86 * 10^-4 kg of helium at 18.0°C. The molar mass of helium is 4.00 g/mol. (a) How many moles of helium are in the tank?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
200
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos