20. Heat and Temperature
Advanced Calorimetry: Equilibrium Temperature with Phase Changes
Problem 17b
Textbook Question
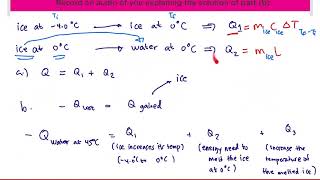
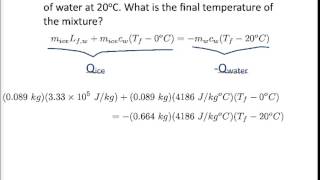
Textbook QuestionA copper calorimeter can with mass 0.100 kg contains 0.160 kg of water and 0.0180 kg of ice in thermal equilibrium at atmospheric pressure. If 0.750 kg of lead at 255°C is dropped into the calorimeter can, what is the final temperature? Assume that no heat is lost to the surroundings.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
15mPlay a video:
548
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos