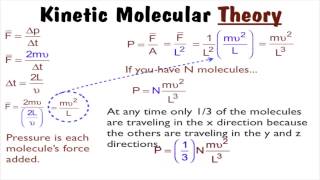
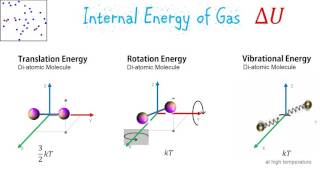
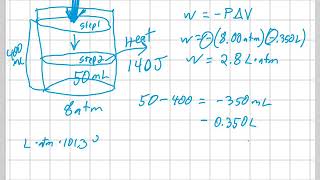21. Kinetic Theory of Ideal Gases
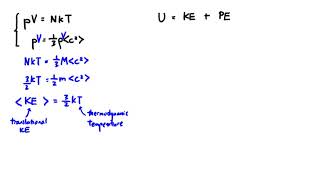
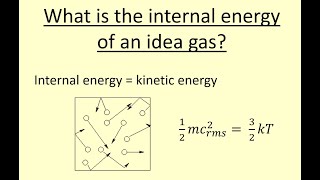
Internal Energy of Gases
Problem 18b
Textbook Question
Textbook QuestionHow much heat does it take to increase the temperature of 1.80 mol of an ideal gas by 50.0 K near room temperature if the gas is held at constant volume and is (b) monatomic?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
185
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos