2. Chemistry
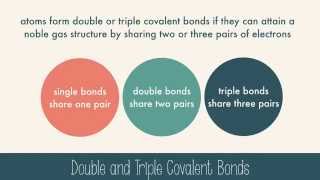
Covalent Bonds
Problem 5a
Textbook Question
Textbook QuestionWhich of these molecules would you predict to have the largest number of polar covalent bonds based on their molecular formulas? a. C2H6O (ethanol) b. C2H6 (ethane) c. C2H4O2 (acetic acid) d. C3H8O (propanol)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
856
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos