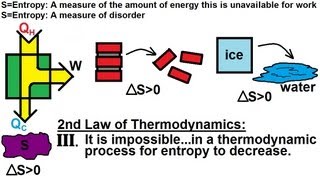
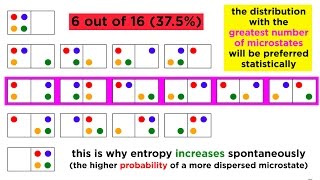
23. The Second Law of Thermodynamics
Entropy and the Second Law of Thermodynamics
Problem 20d
Textbook Question
Textbook QuestionCALC You decide to take a nice hot bath but discover that your thoughtless roommate has used up most of the hot water. You fill the tub with 195 kg of 30.0°C water and attempt to warm it further by pouring in 5.00 kg of boiling water from the stove. (a) Is this a reversible or an irreversible process? Use physical reasoning to explain.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
273
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos