22. The First Law of Thermodynamics
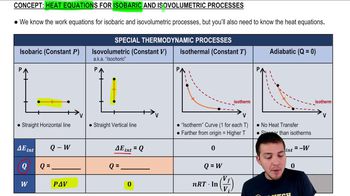
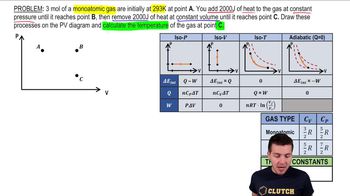
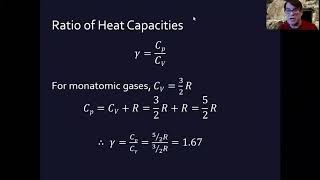
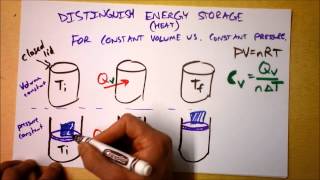
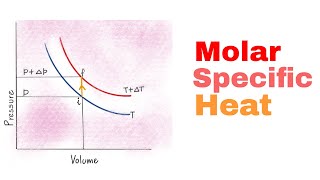
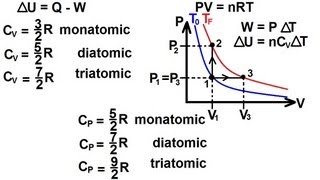
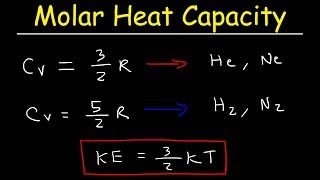
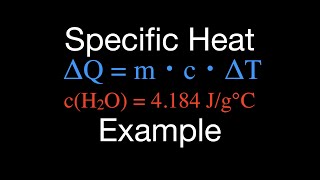
Heat Equations for Special Processes & Molar Specific Heats
Multiple Choice
Multiple ChoiceHow much heat energy is needed to increase the temperature of 5 mol of an ideal diatomic gas by from 273K to 300K if the a) pressure is held constant; b) the volume is held constant?
A
a) 3928 J
b) 2806 J
B
a) 3928 J
b) 1683 J
C
a) 135 J
b) 135 J
D
a) 3.97 × 104 J
b) 4.36 × 104 J
386
views
3
rank
Related Videos
Related Practice
Showing 1 of 9 videos