22. The First Law of Thermodynamics
Cyclic Thermodynamic Processes
Multiple Choice
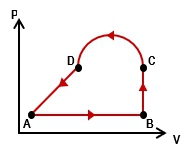
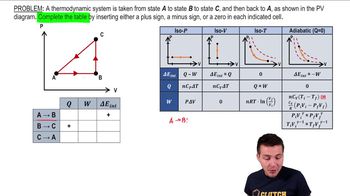
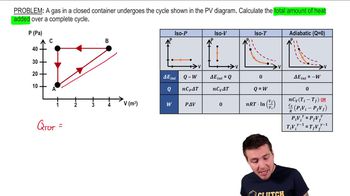
Multiple ChoiceAn ideal gas is taken through the four processes shown below. The changes in internal energy for three of these processes are as follows: ΔEAB = +82 J; ΔEBC = +15 J; ΔEDA = –56 J. Find the change in internal energy for the process from C to D.
A
- 153 J
B
41 J
C
- 41 J
D
Cannot determine
562
views
1
rank
Related Videos
Related Practice