21. Kinetic Theory of Ideal Gases
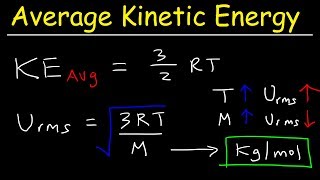
Root-Mean-Square Velocity of Gases
Problem 18e
Textbook Question
Textbook QuestionAt what temperature is the root-mean-square speed of nitrogen molecules equal to the root-mean-square speed of hydrogen molecules at 20.0°C? (Hint: Appendix D shows the molar mass (in g/mol) of each element under the chemical symbol for that element. The molar mass of H2 is twice the molar mass of hydrogen atoms, and similarly for N2.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
389
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos