9. Electrons in Atoms and the Periodic Table
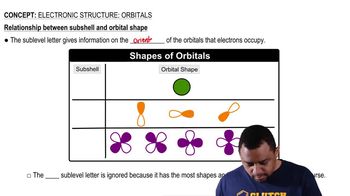
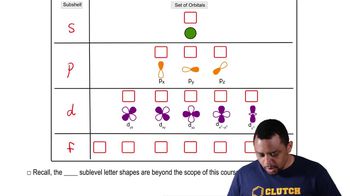
Electronic Structure: Orbitals
9. Electrons in Atoms and the Periodic Table
Electronic Structure: Orbitals
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
Which of the following orbitals possesses the most orbital shapes?
1910views10rank - Multiple Choice
Which of the following statements is false?
a) A set of d orbitals contains 5 orbitals.
b) A set of 4s orbitals would have more energy than a set of 3p orbitals.
c) A set of 3s orbitals would have less energy than a set of 5p orbitals.
d) A set of f orbitals contains 3 orbitals.
e) A set of p orbitals contains 1 orbital.
1911views10rank1comments - Multiple ChoiceIn the modern quantum mechanical model of the atom, electrons are best described as which of the following?1views
- Multiple ChoiceIn atomic theory, what term best describes the region of space around a nucleus where there is a high probability of finding an electron?1views