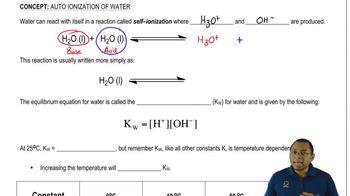
14. Acids and Bases
Auto-Ionization
14. Acids and Bases
Auto-Ionization
Practice this topic
- Textbook QuestionHow is K_w defined, and what is its numerical value at 25 °C?2214views
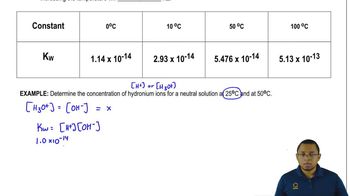
- Textbook QuestionThe dissociation of water into H₃O⁺ and OH⁻ ions depends on temperature. At 0 °C the [H₃O⁺] = 3.38 x 10⁻⁸ M, at 25 °C the [H₃O⁺] = 1.00 x 10⁻⁷ M, and at 50 °C the [H₃O⁺] = 2.34 x 10⁻⁷ M.Is the dissociation of water endothermic or exothermic?2322views
- Textbook QuestionThe dissociation of water into H₃O⁺ and OH⁻ ions depends on temperature. At 0 °C the [H₃O⁺] = 3.38 x 10⁻⁸ M, at 25 °C the [H₃O⁺] = 1.00 x 10⁻⁷ M, and at 50 °C the [H₃O⁺] = 2.34 x 10⁻⁷ M.What is the value of K_w at 0 °C and 50 °C.1825views