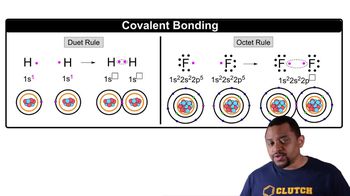
10. Chemical Bonding
Covalent Bonds
10. Chemical Bonding
Covalent Bonds
Practice this topic
- Textbook QuestionWhat is a coordinate covalent bond, and how does it differ from a covalent bond?2160views
- Textbook QuestionIdentify the bonds formed between the following pairs of atoms as either covalent or ionic.d. Zinc and fluorine1809views
- Textbook QuestionWhich of the following contains a coordinate covalent bond? (Hint: How many covalent bonds would you expect the central atom (underlined/bold) to form?) a. PbCl₂ b. Cu (NH₃)₄²⁺ c. NH⁺₄3171views
- Textbook QuestionA compound of gallium with chlorine has a melting point of 77°C and a boiling point of 201°C. Is the compound ionic or covalent? What is a likely formula?2288views
- Multiple ChoiceIn general chemistry, what is the smallest unit of a covalent compound that retains the compound’s chemical identity?3views
- Multiple ChoiceIn a water molecule, what holds the hydrogen atoms to the oxygen atom?4views
- Multiple ChoiceIn the context of covalent bonding, which species does an electron repel due to electrostatic (Coulombic) forces?2views
- Multiple ChoiceWhich of the following compounds is most likely to be covalent (molecular) rather than ionic?3views