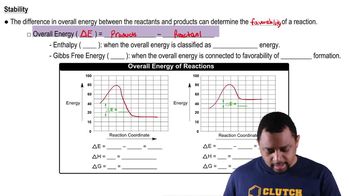
15. Chemical Equilibrium
Energy Diagrams
15. Chemical Equilibrium
Energy Diagrams
Practice this topic
- Multiple Choice
Which of the following statements is true regarding the energy diagram provided?
i. The reaction is endothermic.
ii. The activation energy is +10 kJ.
iii. The reaction releases energy.
iv. The enthalpy of the reaction is –25 kJ.950views4rank - Multiple Choice
Which of the following reactions proceeds the slowest?
Reaction 1 Reaction 2 Reaction 3
818views4rank - Textbook QuestionWhich reaction is faster, one with E_act = +10 kcal/mol(+41.8 kJ/mol) or one with E_act = +5 kcal/mol(+20.9 kJ/mol)? Explain.2016views
- Textbook Questiona. Why do chemical reactions require energy of activation?2469views
- Textbook Questionc. Draw an energy diagram for an exothermic reaction.2442views
- Textbook QuestionSketch an energy diagram for a system in which the forward reaction has E_act = +25 kcal/mol (+105 kJ/mol) and the reverse reaction has E_act = +35 kcal/mol (+146 kJ/mol).Is the forward process endergonic or exergonic?1815views