12. Liquids, Solids, and Intermolecular Forces
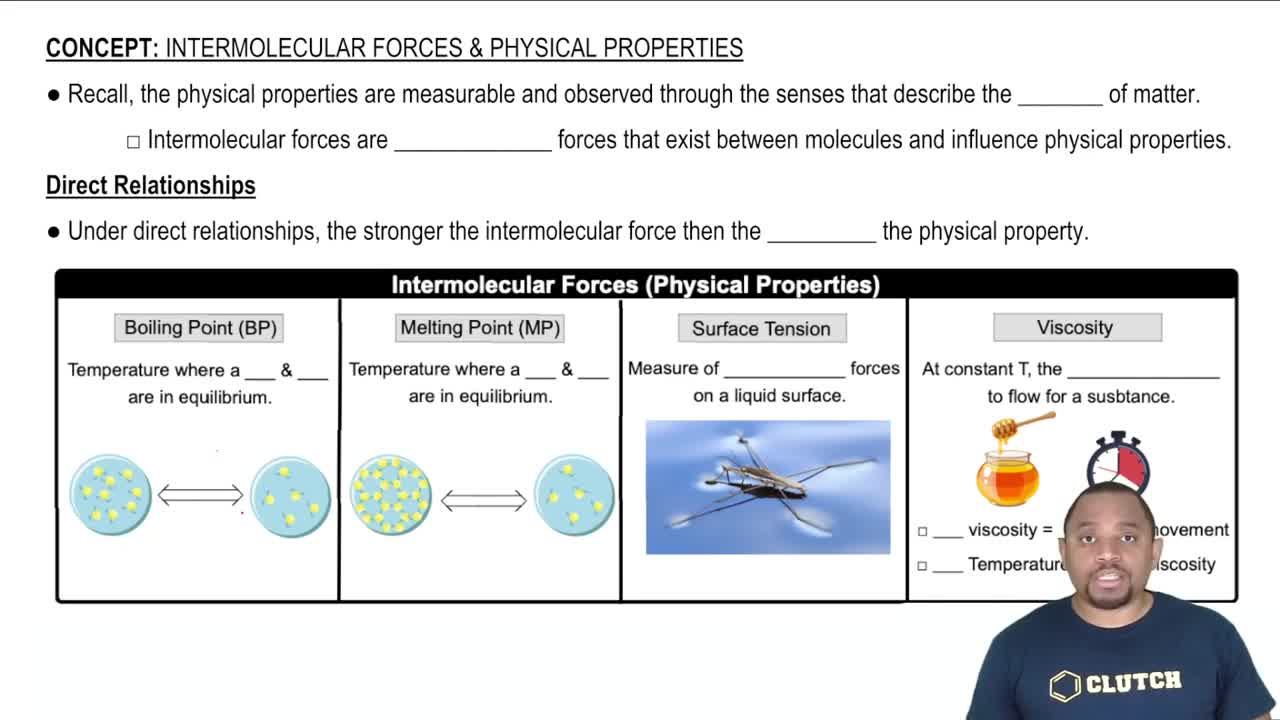
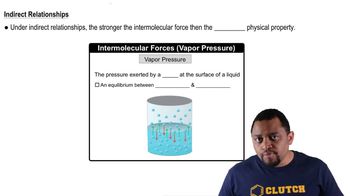
Intermolecular Forces and Physical Properties
12. Liquids, Solids, and Intermolecular Forces
Intermolecular Forces and Physical Properties
Practice this topic
- Multiple Choice
Which of the following will have the lowest boiling point?
1175views1rank - Multiple Choice
Which molecules would most likely cause a liquid to have the lowest viscosity?
770views1rank - Multiple Choice
Which of the following should have the highest surface tension at a given temperature?
998views - Textbook QuestionWould you expect the boiling points to increase or decrease in the following series? Explain.Kr, Ar, Ne1312views
- Textbook QuestionCompare the ∆Hvap values for water, isopropyl alcohol, ether, and ammonia, and order them from lowest to highest. Explain the rank order based on intermolecular attractive forces.1348views
- Textbook QuestionWhat is the effect of pressure on a liquid's boiling point?2479views