Multiple Choice
Based on Rutherford’s gold foil experiment, most of the volume of an atom is occupied by the ____.
 Verified step by step guidance
Verified step by step guidance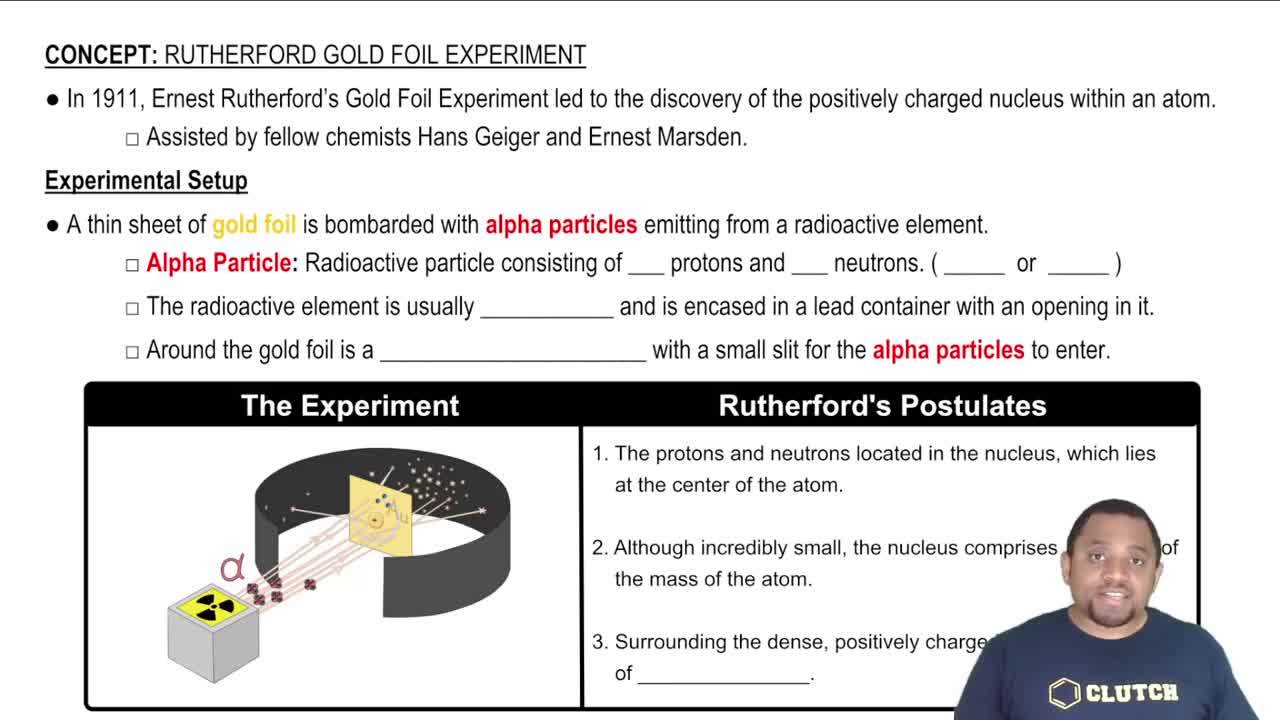


 4:22m
4:22mMaster Rutherford Gold Foil Oil Experiment with a bite sized video explanation from Jules
Start learning