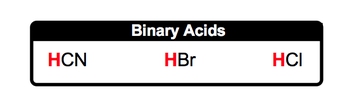
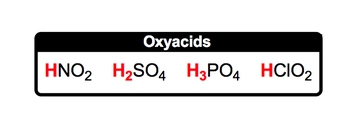
An acid is defined as a covalent compound that typically begins with a hydrogen ion (H+), which is also known as a hydronium ion. Covalent compounds are formed exclusively from nonmetals bonded together. To identify nonmetals, one can refer to the periodic table and the classifications of elements within it.
Common examples of acids include HCl (hydrochloric acid), HNO2 (nitrous acid), and H2SO4 (sulfuric acid). All these acids are covalent, as they consist of nonmetals and start with hydrogen, indicating their acidic nature. While many acids follow this pattern, exceptions do exist. A notable example is acetic acid, which can be represented as CH3COOH. In this case, the hydrogen ion is positioned at the end of the formula, deviating from the typical structure of starting with hydrogen.
Understanding the basic structure of acids sets the foundation for exploring the various types of acids and the rules for naming them, which will be discussed further in subsequent lessons.