Multiple Choice
Which group of elements on the periodic table is known for not reacting (or reacting only very rarely) with other elements under normal conditions?
6
views
 Verified step by step guidance
Verified step by step guidance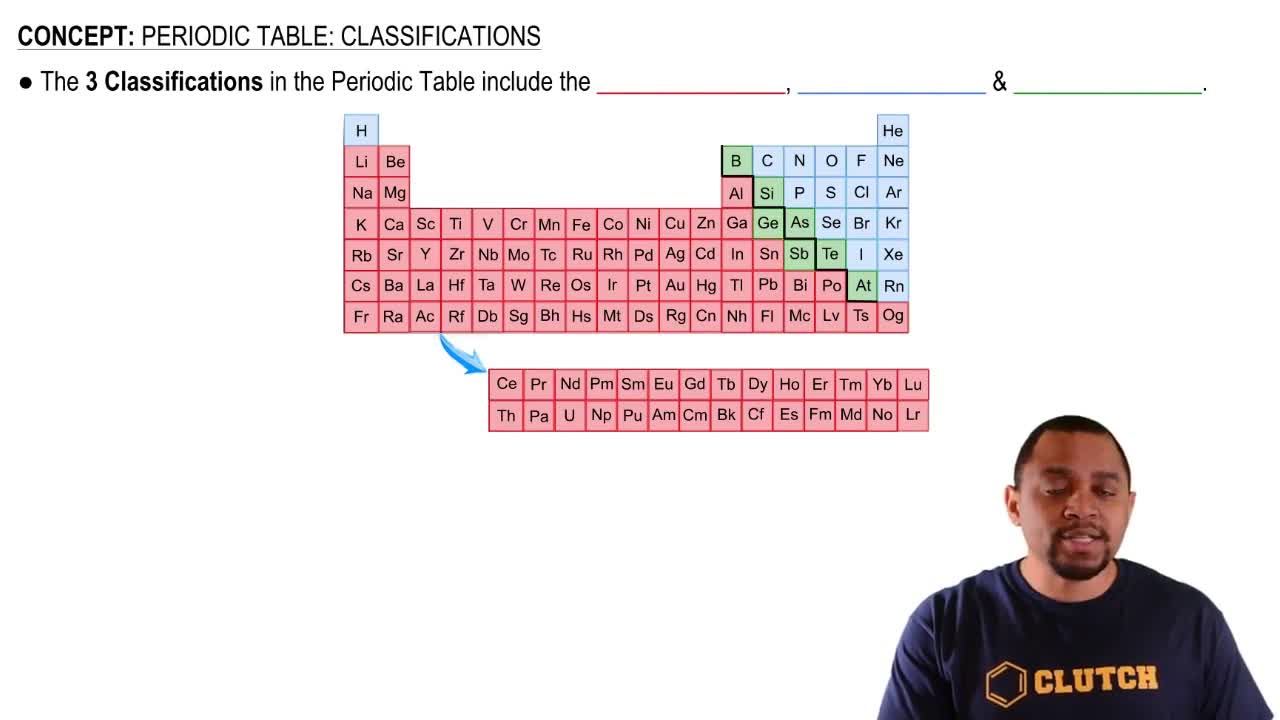



 5:33m
5:33mMaster Periodic Table: Group Names with a bite sized video explanation from Jules
Start learning