Textbook Question
A mixture of nitrogen (N₂) and helium has a volume of 250 mL at 30 °C and a total pressure of 745 mmHg. (8.5, 8.6, 8.7)a. If the partial pressure of helium is 32 mmHg, what is the partial pressure of the nitrogen?
 Verified step by step guidance
Verified step by step guidance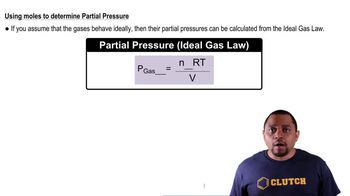



 0:44m
0:44mMaster Dalton's Law: Partial Pressure (Simplified) Concept 1 with a bite sized video explanation from Jules
Start learning