Textbook Question
Which of the following solutions will give rise to a greater osmotic pressure at equilibrium: 5.00 g of NaCl in 350.0 mL water or 35.0 g of glucose in 400.0 mL water? For NaCl, MW = 58.5 amu; for glucose, MW = 180 amu.
 Verified step by step guidance
Verified step by step guidance

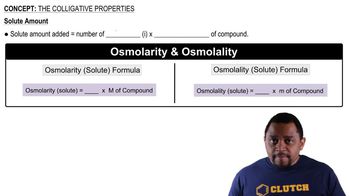

 2:49m
2:49mMaster The Colligative Properties Concept 1 with a bite sized video explanation from Jules
Start learning