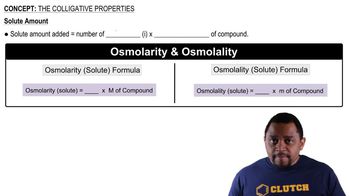
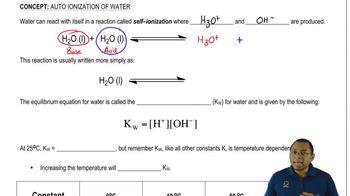
Textbook Question
To prevent accumulation of ice on roads and sidewalks, many municipalities (and home-owners) will apply de-icing compounds to 'melt' the ice by lowering the freezing point.Some de-icing compositions include dyes or colored compounds called indicators. Why?