Textbook Question
The diagram to the right shows plots of vapor pressure versus temperature for a solvent and a solution. What is the approximate boiling-point elevation for the solution?
 Verified step by step guidance
Verified step by step guidance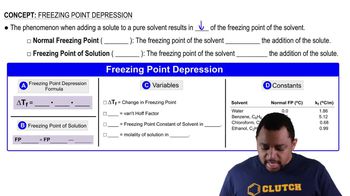


 2:49m
2:49mMaster The Colligative Properties Concept 1 with a bite sized video explanation from Jules
Start learning