A student prepared a stock solution by dissolving 25.00 g of NaOH in enough water to make 150.0 mL solution. The student took 20.0 mL of the stock solution and diluted it with enough water to make 250.0 mL solution. Finally taking 75.0 mL of that solution and dissolving it in water to make 500 mL solution. What is the concentration of NaOH for this final solution? (MW of NaOH:40.00 g/mol).
Table of contents
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 19m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity17m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)11m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 8m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 7m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
13. Solutions
Dilutions
Problem 63
Textbook Question
An aqueous solution that contains 285 ppm of potassium nitrate (KNO₃) is being used to feed plants in a garden. What volume of this solution is needed to prepare 2.0 L of a solution that is 75 ppm in KNO₃?
 Verified step by step guidance
Verified step by step guidance1
Understand that ppm (parts per million) is a way to express concentration, where 1 ppm is equivalent to 1 mg of solute per liter of solution.
Use the dilution equation, which is based on the principle of conservation of mass: \( C_1V_1 = C_2V_2 \), where \( C_1 \) and \( V_1 \) are the concentration and volume of the initial solution, and \( C_2 \) and \( V_2 \) are the concentration and volume of the final solution.
Identify the given values: \( C_1 = 285 \) ppm, \( C_2 = 75 \) ppm, and \( V_2 = 2.0 \) L.
Rearrange the dilution equation to solve for \( V_1 \): \( V_1 = \frac{C_2 \times V_2}{C_1} \).
Substitute the known values into the equation to find \( V_1 \), the volume of the initial solution needed.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Parts Per Million (ppm)
Parts per million (ppm) is a unit of measurement used to describe the concentration of a substance in a solution. It indicates how many parts of a solute are present in one million parts of the solution. For example, a concentration of 285 ppm means that there are 285 grams of solute in one million grams of solution, which is equivalent to 285 mg in 1 L of water.
Recommended video:
Guided course

Kinetic Molecular Theory
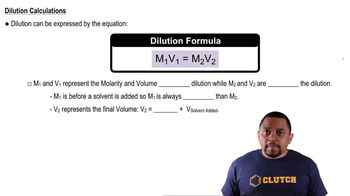
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. The dilution equation, C1V1 = C2V2, relates the initial concentration (C1) and volume (V1) of the concentrated solution to the final concentration (C2) and volume (V2) of the diluted solution. Understanding this concept is crucial for calculating how to achieve a desired concentration from a more concentrated solution.
Recommended video:
Guided course
Dilutions
Concentration Calculation
Calculating concentration involves determining the amount of solute present in a given volume of solution. In this context, to find the volume of the original solution needed to achieve a specific concentration in a new volume, one must apply the principles of concentration and dilution. This requires manipulating the dilution equation to isolate the unknown volume, ensuring accurate preparation of the desired solution.
Recommended video:
Guided course

Calculate Oxidation Numbers
Related Videos
Related Practice
Multiple Choice


