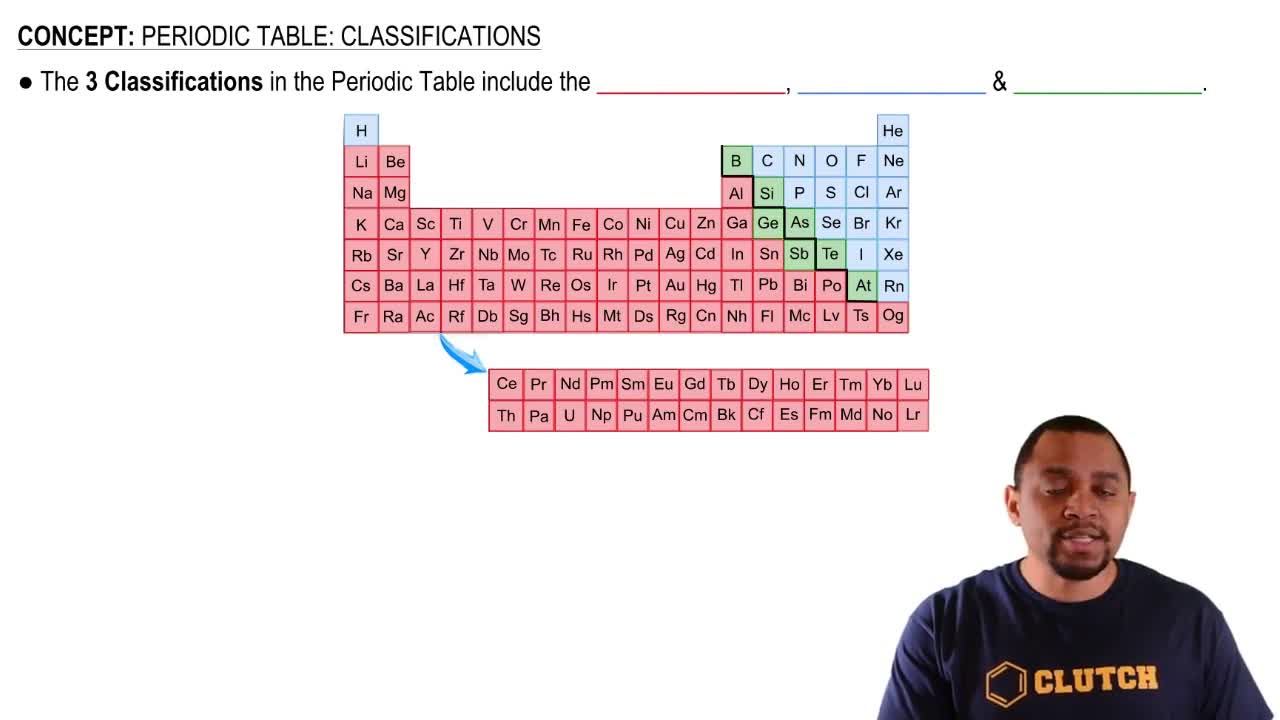
Multiple Choice
Based on the number of protons each element has, which would you expect to be chemically similar?
2066
views
17
rank

 Verified step by step guidance
Verified step by step guidance


 4:01m
4:01mMaster Periodic Table: Symbols Concept with a bite sized video explanation from Jules
Start learning