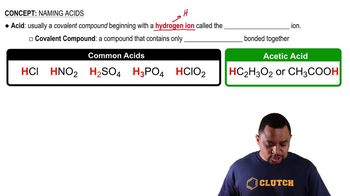
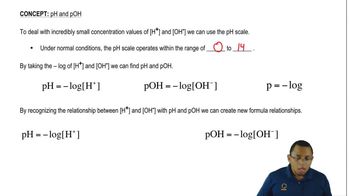Textbook Question
Many allergy medications contain antihistamines, compounds that contain amine groups (R-NH₂, where R refers to an organic functional group). Would you expect these compounds to be acidic, basic or neutral? Explain.One over-the-counter product lists the active ingredient as 'diphenhydramine HCl.' What does this designation mean?