Table of contents
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 19m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity17m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)11m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 8m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 7m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
15. Chemical Equilibrium
Le Chatelier's Principle
Problem 100
Textbook Question
Many carbonate minerals are insoluble in water and appear in water pipes as 'scale.'Why is scale formation typically only a problem in hot water pipes?
 Verified step by step guidance
Verified step by step guidance1
insert step 1> Understand that 'scale' refers to the solid deposits, often composed of calcium carbonate (CaCO_3), that form in water pipes.
insert step 2> Recognize that the solubility of calcium carbonate decreases as the temperature of the water increases.
insert step 3> Consider that in hot water pipes, the water temperature is higher, leading to a decrease in the solubility of calcium carbonate, which causes it to precipitate out of the solution and form scale.
insert step 4> Note that in cold water pipes, the lower temperature means calcium carbonate remains more soluble, reducing the likelihood of scale formation.
insert step 5> Conclude that the reduced solubility of calcium carbonate at higher temperatures is why scale formation is typically a problem in hot water pipes.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility of Carbonates
The solubility of carbonate minerals in water is influenced by temperature and pressure. Generally, as temperature increases, the solubility of gases decreases, while the solubility of some solids, like carbonates, can also decrease. This means that in hot water, carbonate minerals are less likely to remain dissolved, leading to precipitation and scale formation.
Recommended video:
Guided course
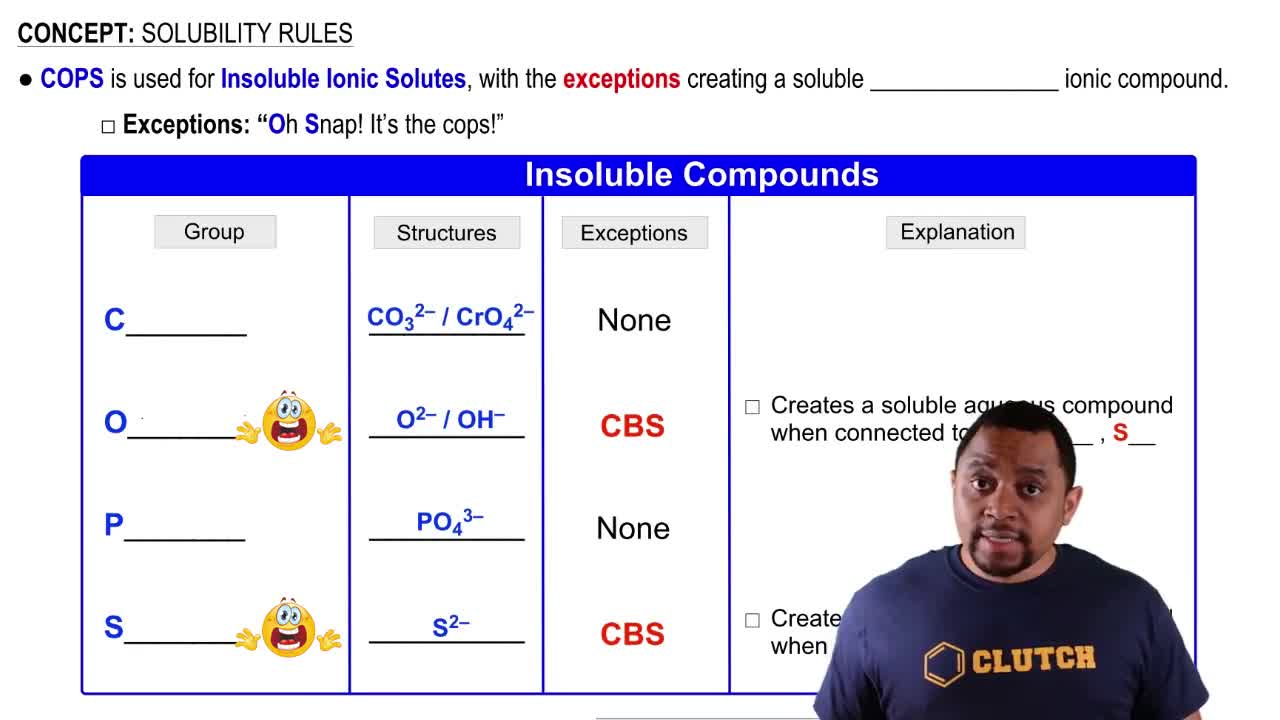
Solubility Rules
Temperature Effects on Water Chemistry
Temperature plays a crucial role in the chemical behavior of water. In hot water pipes, elevated temperatures can increase the rate of evaporation and alter the equilibrium of dissolved minerals. This shift can lead to a higher concentration of dissolved carbonates, which, when cooled or when water evaporates, can precipitate out as scale.
Recommended video:
Guided course

Solubility: Temperature Effect Example 1
Scale Formation Mechanism
Scale formation occurs when dissolved minerals exceed their solubility limit and precipitate out of solution. In hot water pipes, the combination of high temperatures and the presence of dissolved carbonates can lead to rapid scale formation. This buildup can restrict water flow and reduce the efficiency of heating systems, making it a significant maintenance issue.
Recommended video:
Guided course
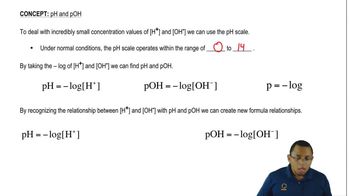
The pH Scale Concept 1
Related Videos
Related Practice
Textbook Question
The reaction CO(g) + H2O(g) ⇌ CO2(g) + H2(g) has ∆H = -9.8 kcal/mol (-41 kJ/mol). Does the amount of H2 in an equilibrium mixture increase or decrease when the temperature is decreased?
2786
views
1
rank


