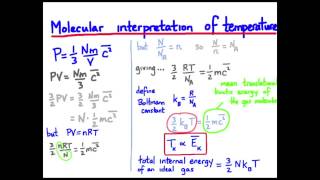
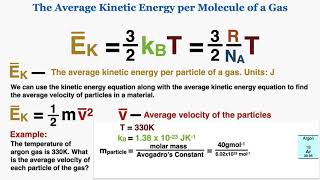
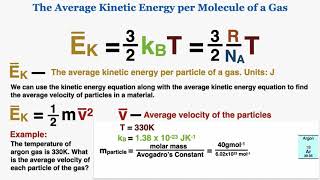
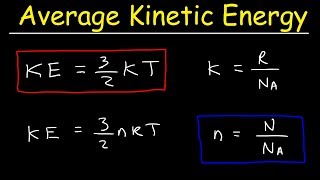
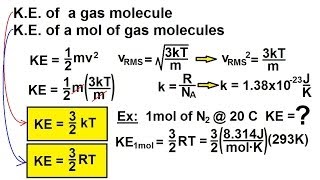
21. Kinetic Theory of Ideal Gases
Average Kinetic Energy of Gases
Learn with other creators
Practice this topic
- Multiple Choice
In a sample of gas, you pick a particle at random. The mass of the particle is 1.67 × 10-27 kg and you measure its speed to be 1600 m/s. If that particle's kinetic energy is equal to the average kinetic energy of the gas particles, what is the temperature of the sample of gas?
844views - Textbook Question
Oxygen (O2) has a molar mass of g/mol. What is the average translational kinetic energy of an oxygen molecule at a temperature of K?
1175views - Textbook Question
A 6.0 m ✕ 8.0 m ✕ 3.0 m room contains air at 20℃. What is the room's thermal energy?
1099views - Textbook Question
The rms speed of the atoms in a 2.0 g sample of helium gas is 700 m/s. What is the thermal energy of the gas?
974views - Textbook Question
Liquid helium boils at 4.2 K. In a flask, the helium gas above the boiling liquid is at the same temperature. What are (a) the mean free path in the gas, (b) the rms speed of the atoms, and (c) the average energy per atom?
800views - Multiple ChoiceThe RMS speed of 65 g of oxygen gas is 254 m/s. Calculate the average kinetic energy of the molecules.458views
- Multiple Choice
Which of the following samples contains particles with the lowest average ?
55views - Multiple Choice
Which of the following samples will have the lowest average kinetic energy per molecule ()?
52views