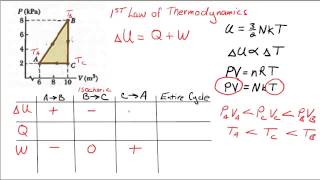
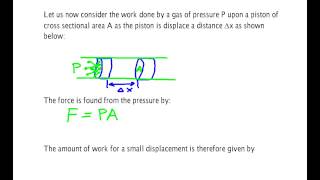
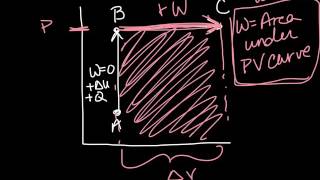
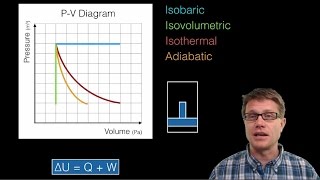
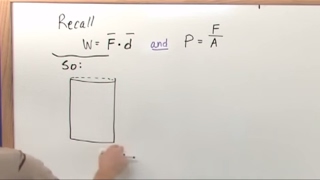22. The First Law of Thermodynamics
PV Diagrams & Work
Learn with other creators
Practice this topic
- Textbook Question
Two moles of an ideal gas are heated at constant pressure from °C to °C. Draw a -diagram for this process.
1308views - Textbook Question
Two moles of an ideal gas are heated at constant pressure from °C to °C. Calculate the work done by the gas.
1384views - Textbook Question
An ideal gas is taken from to on the -diagram shown in Fig. E. During this process, J of heat is added and the pressure doubles. How does the internal energy of the gas at compare to the internal energy at ? Be specific and explain.
1407views - Textbook Question
Figure E shows a -diagram for an ideal gas in which its absolute temperature at is one-fourth of its absolute temperature at . Did heat enter or leave the gas from to ? How do you know?
1625views - Multiple Choice
A PV diagram shows a straight horizontal line from point A at , to point B at , . Which thermodynamic process does this diagram represent?
74views