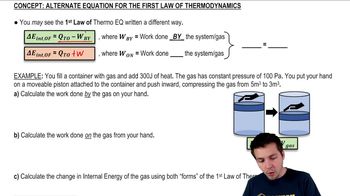
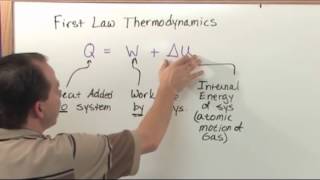
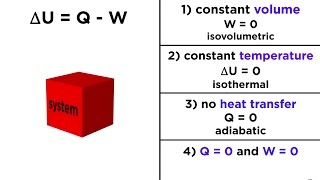
22. The First Law of Thermodynamics
First Law of Thermodynamics
Learn with other creators
Practice this topic
- Multiple Choice
A gas in a cylinder held at a constant pressure 1.80×105 Pa expands from a volume of 1.2 m3 to 1.6 m3. The internal energy of the gas decreases from 4.40×105 J to 3×105 J. How much heat was transferred to the gas?
1155views15rank - Multiple Choice
The internal energy of a system decreases by 500 J, and 230 J of work is done on the system. What is the heat transfer into or out of this system?
1231views13rank - Textbook Question
When water is boiled at a pressure of atm, the heat of vaporization is J/kg and the boiling point is °C. At this pressure, kg of water has a volume of m3, and kg of steam has a volume of m3. Compute the increase in internal energy of the water.
989views - Textbook Question
A gas in a cylinder is held at a constant pressure of Pa and is cooled and compressed from m3 to m3. The internal energy of the gas decreases by J. Does it matter whether the gas is ideal? Why or why not?
929views - Textbook Question
Five moles of an ideal monatomic gas with an initial temperature of °C expand and, in the process, absorb J of heat and do J of work. What is the final temperature of the gas?
1380views - Textbook Question
When water is boiled at a pressure of atm, the heat of vaporization is J/kg and the boiling point is °C. At this pressure, kg of water has a volume of m3, and kg of steam has a volume of m3. Compute the work done when kg of steam is formed at this temperature.
3282views - Multiple Choice
Which statement regarding a closed system is accurate according to the ?
360views - Multiple Choice
Which of the following statements describes the first law of thermodynamics?
288views