20. Heat and Temperature
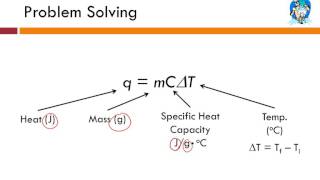
Specific Heat & Temperature Changes
Learn with other creators
Practice this topic
- Multiple Choice
You are given a sample of an unknown metal. You weigh the sample and find that its weight is 29.4N. You add 1.25×104 J of heat energy to the sample and find that its temperature increases from 52°C to 70°C. What is the specific heat of this unknown metal?
839views8rank - Multiple Choiceof heat energy are added to a block of copper that is initially at . What is the final temperature of the copper? The specific heat capacity of copper is .696views
- Textbook Question
A nail driven into a board increases in temperature. If we assume that 60% of the kinetic energy delivered by a 1.80 kg hammer with a speed of 7.80 m/s is transformed into heat that flows into the nail and does not flow out, what is the temperature increase of an 8.00 g aluminum nail after it is struck ten times?
1473views1comments - Textbook Question
While painting the top of an antenna 225 m in height, a worker accidentally lets a 1.00-L water bottle fall from his lunchbox. The bottle lands in some bushes at ground level and does not break. If a quantity of heat equal to the magnitude of the change in mechanical energy of the water goes into the water, what is its increase in temperature?
1227views - Textbook Question
In an effort to stay awake for an all-night study session, a student makes a cup of coffee by first placing a 200-W electric immersion heater in 0.320 kg of water. How much time is required? Assume that all of the heater's power goes into heating the water.
833views - Textbook Question
In very cold weather a significant mechanism for heat loss by the human body is energy expended in warming the air taken into the lungs with each breath. On a cold winter day when the temperature is -20°C, what amount of heat is needed to warm to body temperature (37°C) the 0.50 L of air exchanged with each breath? Assume that the specific heat of air is 1020 J/kg K and that 1.0 L of air has mass 1.3 × 10-3 kg.
1509views