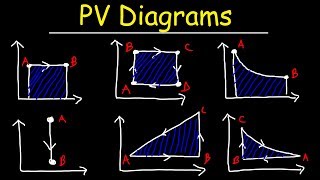
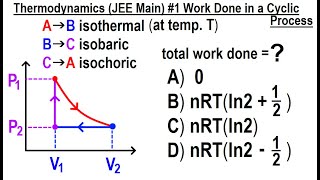
22. The First Law of Thermodynamics
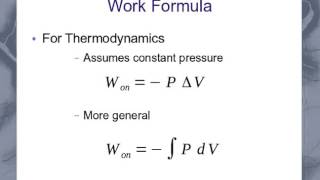
Work Done Through Multiple Processes
Learn with other creators
Practice this topic
- Multiple Choice
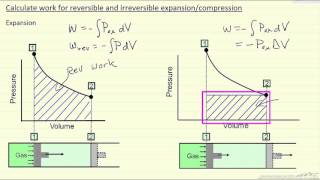
How much work is done on a gas that expands from A to B along the path shown below?
922views4rank2comments - Multiple Choice
A gas with an initial volume of 0.2 m3 is heated at constant volume, and the pressure increases from 2×105 Pa to 5×105. Then, it compresses at constant pressure until it reaches a final volume of 0.12 m3. Draw the two processes in the PV diagram below and find the total work done by the gas.
831views7rank - Multiple ChoiceHow much work is done on the gas in the process shown in the figure? Let , , , and .1132views
- Textbook Question
The -diagram in Fig. E shows a process involving mol of an ideal gas. How much heat had to be added during the process to increase the internal energy of the gas by J?
1564views - Textbook Question
The process shown in the -diagram in Fig. E involves mol of an ideal gas. What was the lowest temperature the gas reached in this process? Where did it occur?
1914views1rank - Textbook Question
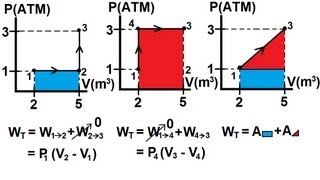
A gas undergoes two processes. In the first, the volume remains constant at m3 and the pressure increases from Pa to Pa. The second process is a compression to a volume of m3 at a constant pressure of Pa. Find the total work done by the gas during both processes.
1698views2rank - Textbook Question
A gas undergoes two processes. In the first, the volume remains constant at m3 and the pressure increases from Pa to Pa. The second process is a compression to a volume of m3 at a constant pressure of Pa. In a -diagram, show both processes.
779views - Multiple ChoiceA proton with an initial speed of 750,000 m/s is brought to rest by an electric field. What was the potential difference that stopped the proton, given that the mass of a proton is approximately 1.67 x 10^-27 kg and its charge is 1.6 x 10^-19 C?312views