23. The Second Law of Thermodynamics
Heat Engines & PV Diagrams
23. The Second Law of Thermodynamics
Heat Engines & PV Diagrams
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
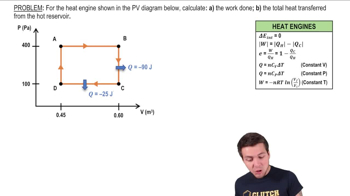
- Multiple ChoiceThe cycle in the figure shows four processes. Process a is an isobaric expansion at . Process b is a constant volume reduction in pressure to . Process c returns the gas to its original state where the volume is . If the gas does of work each cycle, what is the maximum volume of the gas?719views
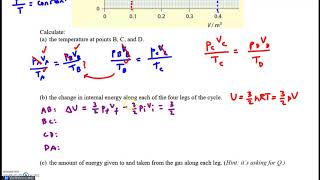
- Multiple ChoiceThe figure shows the cycle of a heat engine that uses a diatomic gas. The temperature at point 1 is . What is the thermal efficiency of the engine?887views
- Multiple Choice
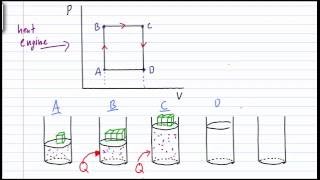
A heat engine follows the cycle shown below. What is the thermal efficiency (%) of this engine?
740views3rank1comments - Textbook Question
A heat engine does 200 J of work per cycle while exhausting 400 J of waste heat. What is the engine's thermal efficiency?
563views - Textbook Question
A Boeing 777 jet engine, the world's largest, has a power output of 82 MW. It burns jet fuel with an energy density of 43 MJ /kg. What is the engine's fuel consumption rate, in kg/s, if its efficiency is 30%?
1468views - Textbook Question
The power output of a car engine running at 2400 rpm is 500 kW. How much (a) work is done and (b) heat is exhausted per cycle if the engine's thermal efficiency is 20%? Give your answers in kJ.
968views - Textbook Question
What are (a) Wout and QH and (b) the thermal efficiency for the heat engine shown in FIGURE EX21.14?
1099views