Multiple Choice
Molecules A and B contain 110 kcal/mol of free energy, and molecules B and C contain 150 kcal/mol of energy. A and B are converted to C and D. What can be concluded?
1644
views
 Verified step by step guidance
Verified step by step guidance
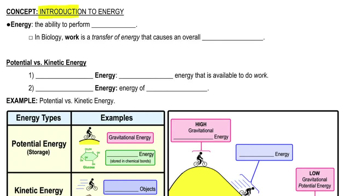


 1:56m
1:56mMaster Chemical Reactions with a bite sized video explanation from Bruce Bryan
Start learning