Enzymes are biological catalysts that facilitate chemical reactions by forming an enzyme-substrate complex. Two primary models explain how enzymes interact with substrates: the lock and key model and the induced fit model. This summary focuses on the lock and key model, which illustrates a rigid and complementary relationship between the enzyme's active site and the substrate.
In the lock and key model, the active site of the enzyme is shaped like a lock, while the substrate is shaped like a key. This perfect fit allows the substrate to bind to the enzyme without any conformational changes. The interaction is akin to a puzzle piece fitting snugly into its corresponding piece, resulting in a stable enzyme-substrate complex. However, this stability can be a drawback, as it may lead to an unchanged or even increased activation energy compared to the uncatalyzed reaction.
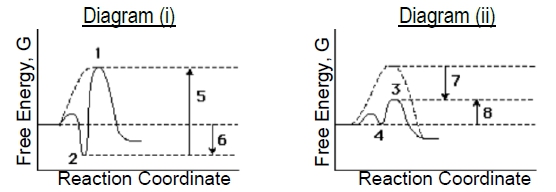
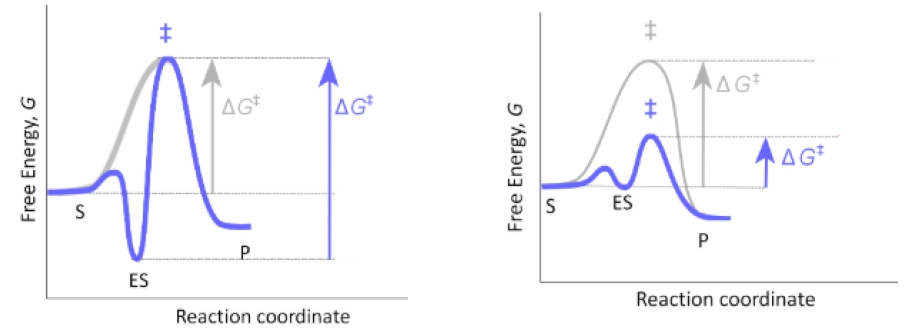
The energy diagram for the lock and key model illustrates this concept. The y-axis represents free energy, while the x-axis represents the reaction coordinate. The dotted line indicates the energy profile of the uncatalyzed reaction, while the red curve represents the enzyme-catalyzed reaction. The activation energy for both reactions is depicted by arrows, showing that the energy barrier for the enzyme-catalyzed reaction remains similar to that of the uncatalyzed reaction. This stabilization of the enzyme-substrate complex can hinder the enzyme's ability to lower the activation energy, which is contrary to the primary function of enzymes.
Ultimately, while the lock and key model provides a foundational understanding of enzyme specificity, it is considered less likely to accurately represent enzyme catalysis due to its potential to stabilize the enzyme-substrate complex excessively. This leads to the need for alternative models, such as the induced fit model, which will be explored further in subsequent discussions.

 1 student found this helpful
1 student found this helpful