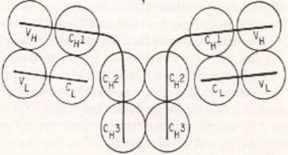
Understanding protein structure is essential in biochemistry, particularly when exploring the tertiary structure, which encompasses the overall three-dimensional shape of a protein. This structure is influenced by the arrangement of secondary structures, including alpha helices, beta strands, beta turns, and loops. These secondary structures are critical components that contribute to the formation of protein motifs and domains.
At the foundation of protein structure lies the amino acid sequence, which is the specific order of amino acids in a polypeptide chain, extending from the N-terminal to the C-terminal. This sequence dictates how the protein will fold and function. The secondary structures arise from interactions between the amino acids, leading to the formation of alpha helices and beta sheets, as well as loops and turns.
As we progress to tertiary structure, it is important to recognize that secondary structures can combine to create super secondary structures, also referred to as motifs. These motifs are specific arrangements of secondary structures that play a role in the protein's function. Furthermore, protein domains are defined as independent folding units within the protein, often associated with specific functions or activities.
In summary, the relationship between amino acid sequence, secondary structures, motifs, and domains is crucial for understanding the complexity of protein tertiary structure. This hierarchical organization not only aids in the comprehension of protein folding but also highlights the functional significance of different structural elements within proteins.