Enzymes are crucial biological catalysts that facilitate chemical reactions in living organisms. They are categorized into six major classes, each defined by the type of reaction they catalyze. These classes are:
- Oxidoreductases: Enzymes that catalyze oxidation-reduction reactions, where the transfer of electrons occurs.
- Transferases: Enzymes that transfer functional groups from one molecule to another.
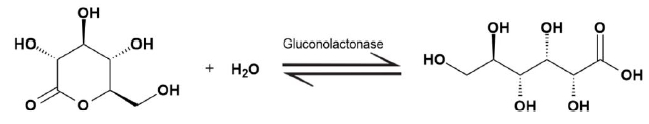
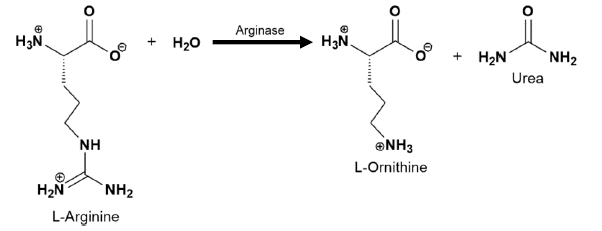
- Hydrolases: Enzymes that catalyze the hydrolysis of various bonds, breaking down molecules by adding water.
- Isomerases: Enzymes that catalyze the rearrangement of atoms within a molecule, converting it into its isomer.
- Lyases: Enzymes that catalyze the addition or removal of groups to form double bonds or break them without hydrolysis or oxidation.
- Ligases: Enzymes that join two molecules together, often using energy derived from ATP.
To remember these classes, one effective mnemonic is the phrase "over the hill," where each letter corresponds to the first letter of each enzyme class. This visualization can help in understanding how enzymes lower the activation energy required for reactions, effectively helping them "get over the hill." Enzymes achieve this by stabilizing the transition state, which is a temporary state during the reaction process.
As you continue your studies, recognizing the specific reactions catalyzed by each enzyme class will be essential for mastering biochemistry concepts. The next focus will be on oxidoreductases, the first class of enzymes, which play a vital role in metabolic processes.