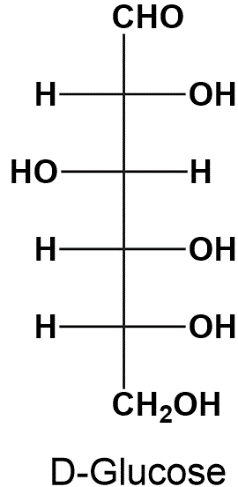
Monosaccharides, the simplest form of carbohydrates, possess chiral centers that can be configured in different ways. Understanding these configurations is crucial in organic chemistry, particularly when studying the structure and function of sugars. There are two primary systems used to designate the configurations of chiral carbons in monosaccharides: the Cahn-Ingold-Prelog system and Fischer's convention.
The Cahn-Ingold-Prelog system utilizes the letters R and S to indicate the configuration of chiral centers. This system is based on the priority of substituents attached to the chiral carbon, where higher atomic number atoms receive higher priority. The configuration is determined by the arrangement of these substituents in three-dimensional space.
On the other hand, Fischer's convention employs the letters D and L to denote the configuration of monosaccharides. This system is particularly useful for classifying sugars based on the orientation of the hydroxyl group (-OH) on the penultimate carbon (the second to last carbon in the chain). If the hydroxyl group is on the right in a Fischer projection, the sugar is designated as D; if it is on the left, it is designated as L.
As you delve deeper into the study of monosaccharides, you will explore each of these systems in detail, starting with the Cahn-Ingold-Prelog system. Understanding these configurations is essential for grasping the biochemical roles of carbohydrates in living organisms.