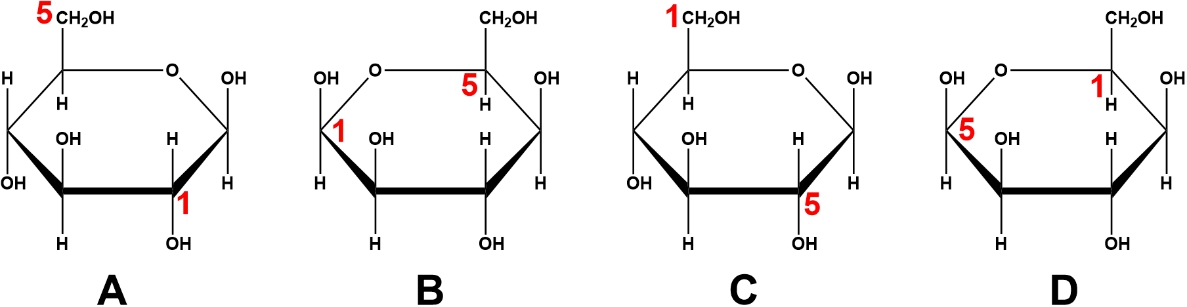
Cyclic monosaccharides are crucial in biochemistry, particularly because most carbohydrates with five or more carbon atoms exist in cyclic forms in biological solutions. While monosaccharides can form various ring structures, the most common and stable configurations are five-membered and six-membered rings. Monosaccharides that form five-membered rings are known as furanoses, while those that form six-membered rings are called pyranoses.
The terminology stems from the structural resemblance of these rings to the compounds furan and pyran, respectively. A furanose ring consists of four carbon atoms and one oxygen atom, making it a five-membered ring, while a pyranose ring contains five carbon atoms and one oxygen atom, forming a six-membered ring. This distinction is important as it highlights the role of oxygen in these cyclic structures.
For example, the formation of a furanose from the linear form of D-fructose occurs when the hydroxyl group on carbon 5 interacts with the ketone group on carbon 2. This reaction leads to the creation of D-fructofuranose, which resembles the furan structure. Similarly, D-glucose can cyclize into a pyranose form when the hydroxyl group on carbon 5 interacts with the aldehyde group on carbon 1, resulting in D-glucopyranose, which resembles the pyran structure.
Understanding these cyclic forms is essential for grasping the behavior and function of carbohydrates in biological systems, as they play a significant role in various biochemical processes. As we delve deeper into the study of cyclic monosaccharides, we will explore their reactions and significance in greater detail.