Understanding monosaccharide stereochemistry is essential for grasping the complexities of carbohydrates. This topic builds on foundational concepts from organic chemistry, so a review of previous organic chemistry lessons may be beneficial if any terminology feels unfamiliar.
Monosaccharides are often represented using Fischer projections, which are two-dimensional depictions of three-dimensional sugar molecules. In Fischer projections, horizontal bonds extend out of the page as wedges, while vertical bonds go behind the page. This representation helps visualize the stereochemistry of these molecules effectively.
Key terms in this discussion include:
Constitutional Isomers: These are molecules that share the same chemical formula but differ in the connectivity of their atoms. For example, glyceraldehyde and dihydroxyacetone (DHA) are constitutional isomers.
Stereoisomers: These molecules also have the same chemical formula but differ in their three-dimensional arrangement. The prefix "stereo" indicates this spatial difference. An example is the variation in the spatial arrangement of hydroxyl groups in certain sugars.
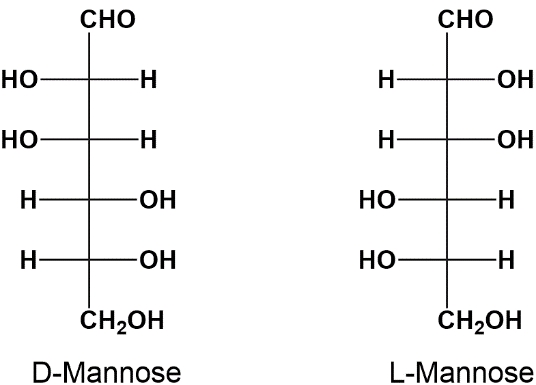
Enantiomers: A specific type of stereoisomer, enantiomers are non-superimposable mirror images of each other. A relatable analogy is the left and right hands, which cannot perfectly overlap when oriented in the same direction. D-glyceraldehyde and L-glyceraldehyde serve as classic examples of enantiomers.
Diastomers: Another category of stereoisomers, diastereomers are not mirror images of each other. They differ in their three-dimensional configurations, which can be visualized through their distinct spatial arrangements.
This overview of monosaccharide stereochemistry highlights the importance of understanding the relationships between different types of isomers, particularly in the context of carbohydrate chemistry. A solid grasp of these concepts will enhance your comprehension of more complex biochemical processes involving sugars.